Heart failure with reduced ejection fraction (HFrEF) is a major type of heart failure, and the China HF Study showed that 42% of heart failures in China are HFrEF, although several standard therapeutic classes of drugs are available for HFrEF and have reduced the risk of death and hospitalization for heart failure to some extent. However, patients are at high risk of recurrent heart failure worsening events, mortality remains at around 25% and prognosis remains poor. Therefore, there is still an urgent need for new therapeutic agents in the treatment of HFrEF, and Vericiguat, a novel soluble guanylate cyclase (sGC) stimulator, was studied in the VICTORIA study to assess whether Vericiguat could improve the prognosis of patients with HFrEF. The study is a multicenter, randomized, parallel-group, placebo-controlled, double-blind, event-driven, phase III clinical outcomes study. Conducted under the auspices of the VIGOUR Center in Canada in collaboration with the Duke Clinical Research Institute, 616 centers in 42 countries and regions, including Europe, Japan, China and the United States, participated in the study. Our cardiology department was honored to participate. A total of 5,050 patients with chronic heart failure aged ≥18 years, NYHA class II-IV, EF <45%, with elevated natriuretic peptide (NT-proBNP) levels within 30 days prior to randomization, and who had been hospitalized for heart failure within 6 months prior to randomization or had diuretics administered intravenously for heart failure within 3 months prior to randomization were enrolled in the study, all receiving ESC, AHA/ACC, and national/ region specific guidelines recommended standard of care. Patients were randomized in a 1:1 ratio to two groups and were given Vericiguat (n=2526) and placebo (n=2524) on top of standard therapy, respectively.
The primary endpoint of the study was the composite endpoint of cardiovascular death or first heart failure hospitalization; secondary endpoints included components of the primary endpoint, first and subsequent heart failure hospitalizations (first and recurrent events), the composite endpoint of all-cause death or heart failure hospitalization, and all-cause death. At a median follow-up of 10.8 months, there was a relative 10% reduction in the primary endpoint of cardiovascular death or first heart failure hospitalization in the Vericiguat group compared with the placebo group.
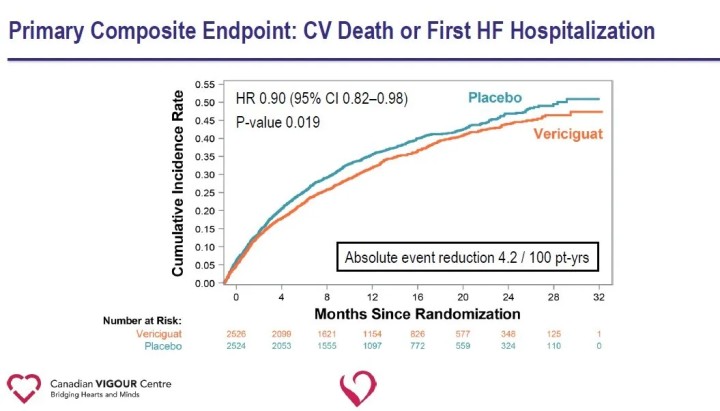
Analysis of secondary endpoints showed a significant reduction in heart failure hospitalization (HR 0.90) and a significant reduction in the composite endpoint of all-cause death or heart failure hospitalization (HR 0.90) in the Vericiguat group compared with the placebo group.
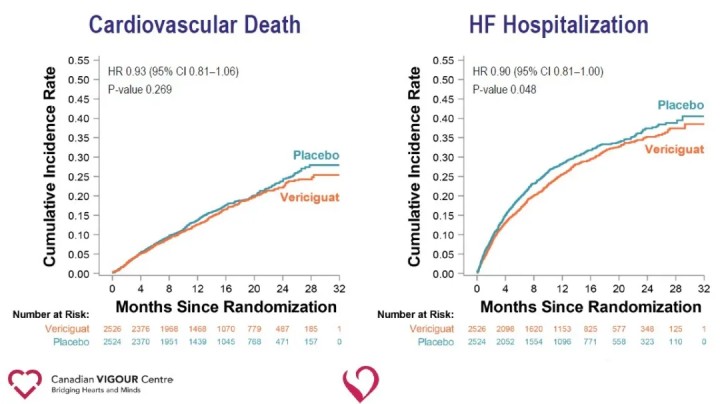
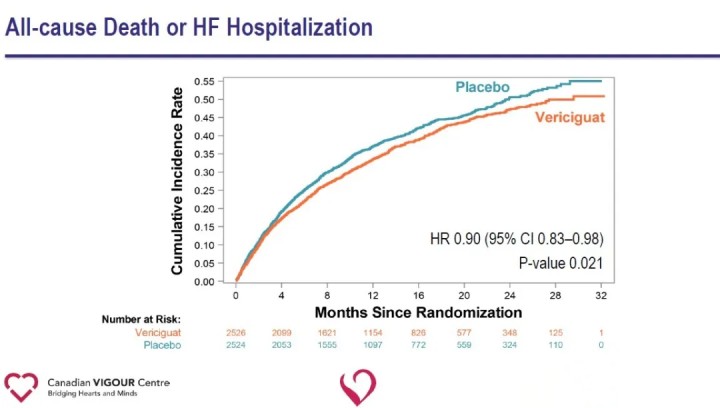
The results of the study suggest that the addition of Vericiguat to standard treatment of heart failure significantly reduces the recent occurrence of worsening heart failure events and reduces the risk of the composite endpoint of cardiovascular death or hospitalization for heart failure in patients with HFrEF. The ability of Vericiguat to reduce the risk of the composite endpoint of cardiovascular death or heart failure hospitalization in patients with high-risk heart failure provides a new therapeutic avenue for heart failure and opens new pathways for future exploration of cardiovascular disease. Vericiguat is not currently approved for marketing. The safety, efficacy and cost effectiveness of the drug still need to be further tested in the market.
Post time: Feb-09-2022
