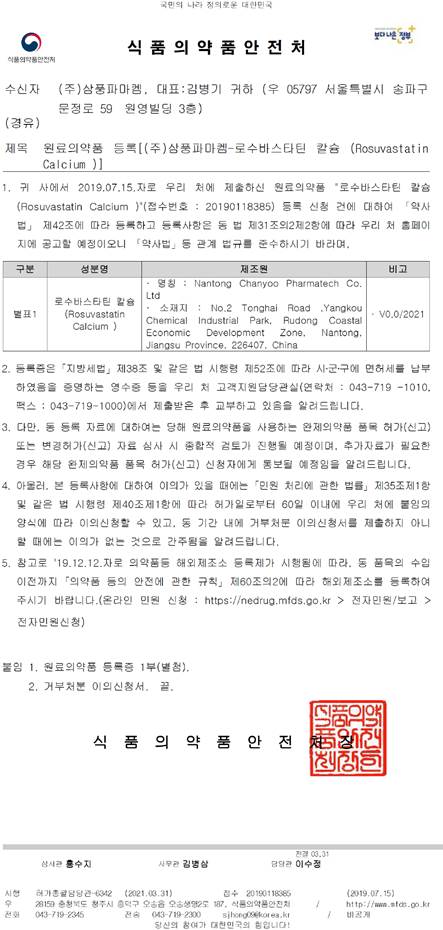
Recently, Nantong Chanyoo have made another milestone in the history! With the efforts for more than one year, the first KDMF of Chanyoo has got approved by MFDS. As the biggest manufacturer of Rosuvastatin Calcium in China, we wish to open a new chapter in Korea market. And more products would be launched in the next few years.
Also, you could refer and find our information from this website:
https://nedrug.mfds.go.kr/pbp/CCBAC03
Rosuvastatin calcium is suitable for the adjuvant treatment of patients with primary hypercholesterolemia (type IIa, including heterozygous familial hypercholesterolemia) or mixed lipemia (type IIb) when dieting or exercise therapy is not ideal . Rosuvastatin calcium is a selective 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor. By inhibiting HMG-CoA reductase, it reduces liver cell synthesis and storage of cholesterol, thereby Lower blood total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels.
According to Newport data, from June 30, 2018 to June 30, 2019, the global sales of rosuvastatin calcium preparations were US$3.461 billion, a year-on-year decrease of 13%; the global sales of rosuvastatin calcium raw materials were 151.09 tons , A year-on-year increase of 14%
We, Changzhou Pharmaceutical Factory (subsidiary manufacturer: Nantong Chanyoo Pharmatech Co., Ltd.) devoted ourself to the largest supplier of Rosuvastatin API.
Post time: Apr-20-2021