Tofacitinib citrate is a prescription drug (trade name Xeljanz) originally developed by Pfizer for a class of oral Janus kinase (JAK) inhibitors. It can selectively inhibit JAK kinase, block JAK/STAT pathways, and thereby inhibit cell signal transduction and Related gene expression and activation, used to treat rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and other immune diseases.
The drug includes three dosage forms: tablets, sustained-release tablets and oral solutions. Its tablets were first approved by the FDA in 2012, and the sustained-release dosage form was approved by the FDA in February 2016. It is the first to treat rheumatoid joints. Yan is a JAK inhibitor taken orally once a day. In December 2019, a new indication for sustained-release drugs was approved again for moderate to severe active ulcerative colitis (UC). In addition, the current phase 3 clinical trials for plaque psoriasis have been completed, and another six phase 3 clinical trials are in progress, involving active psoriatic arthritis, juvenile idiopathic arthritis, etc. Kind of indications. The advantages of sustained-release tablets that are long-acting and only need to be taken once a day are conducive to the management and control of patients’ diseases.
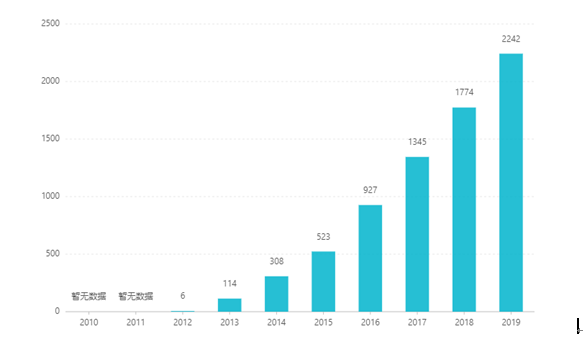
Since its listing, its sales have increased year after year, reaching US$2.242 billion in 2019. In China, the tablet dosage form was approved for marketing in March 2017, and entered the medical insurance category B catalog through negotiations in 2019. The latest winning bid is RMB 26.79. However, due to the high technical barriers of sustained-release preparations, this dosage form has not yet been marketed in China.
JAK kinase plays an important role in inflammation, and its inhibitors have been shown to treat certain inflammatory and autoimmune diseases. Up to now, 7 JAK inhibitors have been approved globally, including Leo Pharma’s Delgocitinib, Celgene’s Fedratinib, AbbVie’s upatinib, Astellas’s Pefitinib, Eli Lilly’s Baritinib And Novartis’s Rocotinib. However, only tofacitinib, baritinib and rocotinib are approved in China among the above-mentioned drugs. We look forward to Qilu’s “Tofatib Citrate Sustained Release Tablets” being approved as soon as possible and benefiting more patients.
In China, the original research tofacitib citrate was approved by the NMPA in March 2017 for the treatment of adult RA patients with insufficient efficacy or intolerance to methotrexate, under the trade name Shangjie. According to data from Meinenet, the sales of tofacitib citrate tablets in China’s public medical institutions in 2018 were 8.34 million yuan, which was far lower than its global sales. A large part of the reason is the price. It is reported that Shangjie’s initial retail price was 2085 yuan (5mg*28 tablets), and the monthly cost was 4170 yuan, which is not a small burden for ordinary families.
However, it is worth celebrating that tofacitib was included in the 2019 “National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List” by the National Medical Insurance Administration after negotiations in November 2019. It is reported that the monthly fee will be reduced to below 2,000 yuan after the price cut is negotiated, which will greatly improve the availability of the drug.
In August 2018, the Patent Reexamination Board of the State Intellectual Property Office made a review decision No. 36902 request for invalidation, and declared invalid the core patent of Pfizertofatib, the compound patent, on the grounds of insufficient disclosure of the specification. However, the patent of Pfizertofatiib crystal form (ZL02823587.8, CN1325498C, application date 2002.11.25) will expire in 2022.
The Insight database shows that, in addition to the original research, five generic drugs of Chia Tai Tianqing, Qilu, Kelun, Yangtze River, and Nanjing Chia Tai Tianqing have been approved for marketing in the domestic tofacitinib tablet formulations. However, for the sustained-release tablet type, only the original research Pfizer submitted a marketing application on May 26. Qilu is the first domestic company to submit a marketing application for this formulation. In addition, CSPC Ouyi is in the BE trial stage.
Changzhou Pharmaceutical Factory (CPF) is a leading pharmaceutical manufacturer of APIs, finished formulations in China, which located in Changzhou, Jiangsu province. CPF have been founded in 1949. We have devoted in Tofacitinib Citrate from 2013, and submitted DMF already. We have registered in many countries, and could support you with best documents support for Tofacitinib Citrate.
Post time: Jul-23-2021