100% Original Factory Sglt2 Inhibitor Canagliflozin - Sofosbuvir – CPF
100% Original Factory Sglt2 Inhibitor Canagliflozin - Sofosbuvir – CPF Detail:
| 索非布韦 | Sofosbuvir | 1190307-88-0 | In-House |
| SFBM | 1256490-31-9 | In-House | |
| SFB-8 | 874638-80-9 | In-House | |
| SFB-10 | 817204-32-3 | In-House | |
| SFBA-1 | 863329-66-2 | In-House | |
| SFBMA | 1334513-02-8 | In-House |
Generic Name: sofosbuvir (soe FOS bue vir)
Brand Names: Sovaldi
Sofosbuvir is an antiviral medicine that is used to treat chronic hepatitis C in adults and children who are at least 3 years old and an HCV RNA replication inhibitor with an EC50 of 92 nM.
Sofosbuvir must be given in combination with other antiviral medications (usually ribavirin with or without peginterferon alfa). Sofosbuvir should not be used alone.
Sofosbuvir treats specific genotypes of hepatitis C, and only in certain people. Use only the medications prescribed for you. Do not share your medicine with other people.
Sofosbuvir is sometimes used in people who also have HIV, or people who have liver cancer and are going to have a liver transplant. This medicine is not a treatment for HIV or AIDS.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
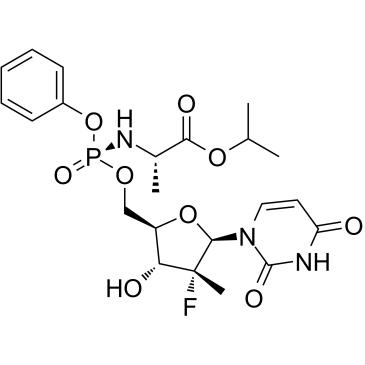
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
It is a great way to further improve our products and repair. Our mission is always to create innovative products to prospects with a superior expertise for 100% Original Factory Sglt2 Inhibitor Canagliflozin - Sofosbuvir – CPF , The product will supply to all over the world, such as: Sierra Leone, Hongkong, Malta, With the first-class products, excellent service, fast delivery and the best price, we have won highly praise foreign customers'. Our products have been exported to Africa, the Middle East, Southeast Asia and other regions.
It can be said that this is a best producer we encountered in China in this industry, we feel lucky to work with so excellent manufacturer.











