2020 Latest Design Apo Hydrochlorothiazide - Pitavastatin Calcium – CPF
2020 Latest Design Apo Hydrochlorothiazide - Pitavastatin Calcium – CPF Detail:
| 匹伐他汀钙 | Pitavastatin | 147526-32-7 | In-House |
| PI-5 | 3800-06-4 | In-House | |
| PI-6 | 145516-11-4 | In-House | |
| PI-7 | 121660-11-5 | In-House |
Description
Pitavastatin Calcium (NK-104 hemicalcium) is a potent hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitor. Pitavastatin Calcium (NK-104 hemicalcium) inhibits cholesterol synthesis from acetic acid with an IC50 of 5.8 nM in HepG2 cells. Pitavastatin Calcium is an efficient hepatocyte low-density lipoprotein-cholesterol (LDL-C) receptor inducer. Anti-cancer activity.
Background
Pitavastatin Calcium is a competitive inhibitor of the enzyme HMGCR (HMG-CoA reductase) results in a reduction in LDL cholesterol synthesis. Alternate studies show that pitavastatin can suppress oxygen production in endothelial cells by inhibiting NADPH oxidase. In addition, pitavastatin reduces the expression of eNOS mRNA while increasing the NO dependent response stimulated by acetylcholine and the calcium ionophore, A23187. Furthermore, pitavastatin inhibits the up-regulation of conductance calcium-activated potassium channels by lowering cholesterol levels in cells.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
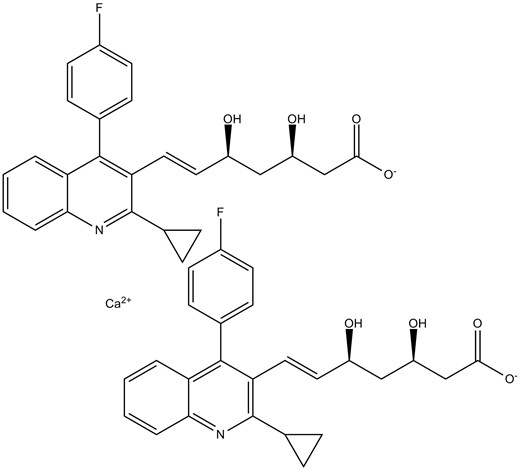
Chemical structure
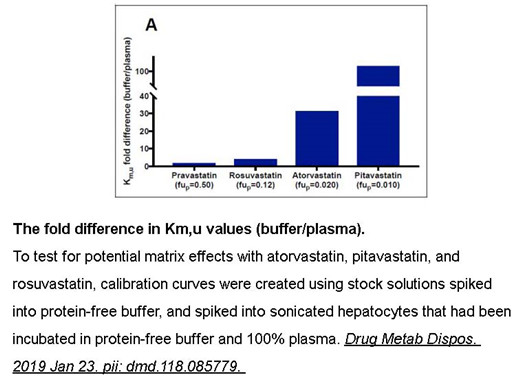
Related Biological Data
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:
Related Product Guide:
We take pleasure in an extremely fantastic standing among the our prospects for our great product top quality, competitive cost and the finest support for 2020 Latest Design Apo Hydrochlorothiazide - Pitavastatin Calcium – CPF , The product will supply to all over the world, such as: Ukraine, Zimbabwe, European, Since always, we adhering to the "open and fair, share to get, the pursuit of excellence, and creation of value"values, adhere to the"integrity and efficient, trade-oriented, best way , best valve" business philosophy. Together with our all over the world have branches and partners to develop new business areas, maximum common values. We sincerely welcome and together we share in global resources, opening up new career together with the chapter.
The manufacturer gave us a big discount under the premise of ensuring the quality of products, thank you very much, we will select this company again.










