2020 New Style Asunaprevir Daclatasvir - Lenalidomide – CPF
2020 New Style Asunaprevir Daclatasvir - Lenalidomide – CPF Detail:
Description
Lenalidomide (CC-5013) is a derivative of Thalidomide and an orally active immunomodulator. Lenalidomide (CC-5013) is a ligand of ubiquitin E3 ligase cereblon (CRBN), and it causes selective ubiquitination and degradation of two lymphoid transcription factors, IKZF1 and IKZF3, by the CRBN-CRL4 ubiquitin ligase. Lenalidomide (CC-5013) specifically inhibits growth of mature B-cell lymphomas, including multiple myeloma, and induces IL-2 release from T cells.
Background
Lenalidomide (also known as CC-5013), an oral derivative of thalidomide, is an antineoplastic agent exhibiting antitumor activity through a variety of mechanisms, including immune system activation, angiogenesis inhibition, and direct antineoplastic effects. It has been extensively studied for the treatment of multiple myeloma and myelodysplastic syndrome as well as lymphoproliferative disorders including chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma. According to recent studies, Lnalidomide promotes and restores immune system function in CLL patients by inducing an overexpression of costimulatory molecules in leukemic lymphocytes to restore the humoral immunity and immunoglobulins production as well as improving the ability of T cells and leukemic cells to form synapses with T lymphocytes.
Reference
Ana Pilar Gonzalez-Rodriguez, Angel R. Payer, Andrea Acebes-Huerta, Leticia Hergo-Zapico, Monica Villa-Alvarez, Esther Gonzalez-Garcia, and Segundo Gonzalez. Lenalidomide and chronic lymphocytic leukemia. BioMed Research International 2013.
In Vitro
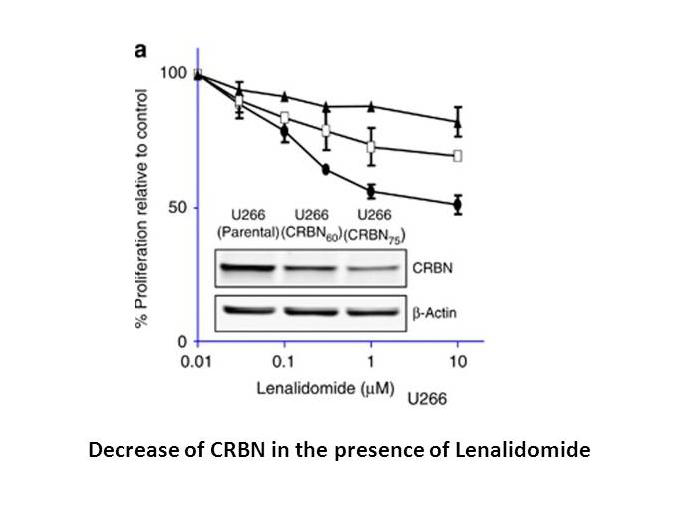
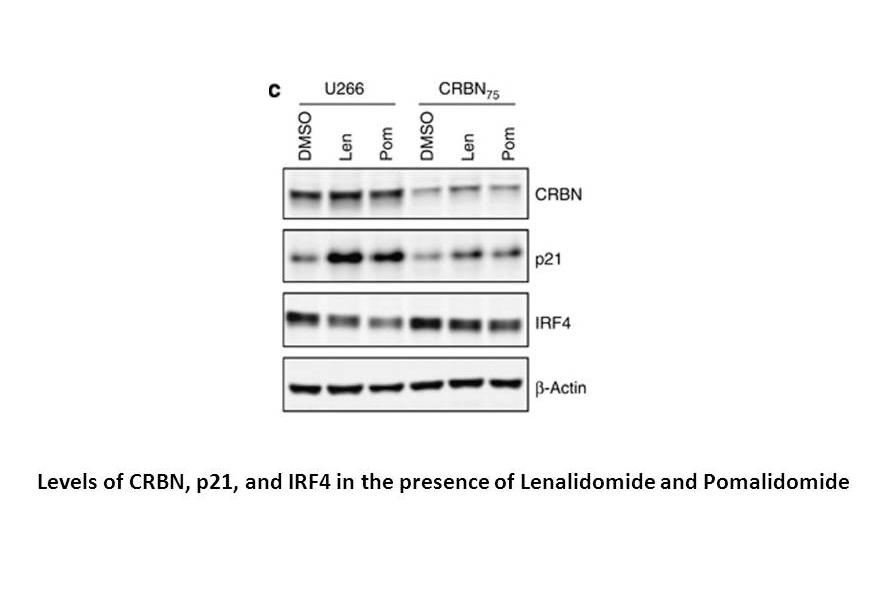
Lenalidomide is potent in stimulating T cell proliferation and IFN-γ and IL-2 production. Lenalidomide has been shown to inhibit production of pro inflammatory cytokines TNF-α, IL-1, IL-6, IL-12 and elevate the production of anti-inflammatory cytokine IL-10 from human PBMCs. Lenalidomide downregulates the production of IL-6 directly and also by inhibiting multiple myeloma (MM) cells and bone marrow stromal cells (BMSC) interaction, which augments the apoptosis of myeloma cells[2]. Dose-dependent interaction with the CRBN-DDB1 complex is observed with Thalidomide, Lenalidomide and Pomalidomide, with IC50 values of ~30 μM, ~3 μM and ~3 μM, respectively, These reduced CRBN expression cells (U266-CRBN60 and U266-CRBN75) are less responsive than the parental cells to antiproliferative effects Lenalidomide across a dose-response range of 0.01 to 10 μM[3]. Lenalidomide, a thalidomide analog, functions as a molecular glue between the human E3 ubiquitin ligase cereblon and CKIα is shown to induce the ubiquitination and degradation of this kinase, thus presumably killing leukemic cells by p53 activation.
The toxicity of Lenalidomide doses up to 15, 22.5, and 45 mg/kg via IV, IP, and PO routes of administration. Limited by solubility in our PBS dosing vehicle, these maximum achievable Lenalidomide doses are well tolerated with the exception of one mouse death (of four total dosed) at the 15 mg/kg IV dose. Notably, no other toxicities are observed in the study at IV doses of 15 mg/kg (n=3) or 10 mg/kg (n=45) or at any other dose level through IV, IP, and PO routes.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
Chemical structure
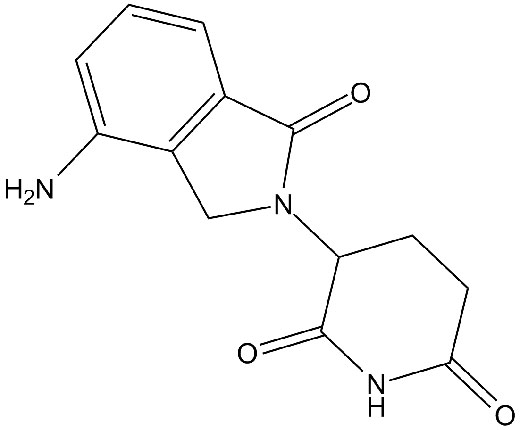
Related Biological Data
Related Biological Data
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
"Sincerity, Innovation, Rigorousness, and Efficiency" may be the persistent conception of our organization to the long-term to build together with shoppers for mutual reciprocity and mutual advantage for 2020 New Style Asunaprevir Daclatasvir - Lenalidomide – CPF , The product will supply to all over the world, such as: Bangkok, Philippines, moldova, Our company is working by the operation principle of "integrity-based, cooperation created, people oriented, win-win cooperation". We hope we can have a friendly relationship with businessman from all over the world.
Goods just received, we are very satisfied, a very good supplier, hope to make persistent efforts to do better.










