China Wholesale Rosuvastatin Calcium Ip Quotes - Ledipasvir Actone /PVP – CPF
China Wholesale Rosuvastatin Calcium Ip Quotes - Ledipasvir Actone /PVP – CPF Detail:
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
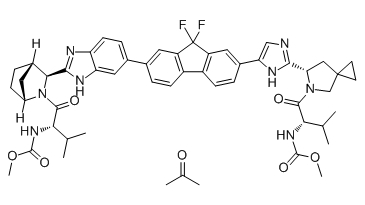
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We take "customer-friendly, quality-oriented, integrative, innovative" as objectives. "Truth and honesty" is our administration ideal for China Wholesale Rosuvastatin Calcium Ip Quotes - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Czech, Lithuania, Iraq, We solution have passed through the national skilled certification and been well received in our key industry. Our specialist engineering team will often be ready to serve you for consultation and feedback. We are able to also provide you with no cost samples to meet your needs. Best efforts will be produced to offer you the very best service and solutions. For anyone who is considering our business and solutions, please speak to us by sending us emails or get in touch with us right away. As a way to know our products and enterprise. lot more, you'll be able to come to our factory to find out it. We will constantly welcome guests from around the globe to our firm. o build enterprise. elations with us. Please really feel absolutely free to make contact with us for small business and we believe we will share the top trading practical experience with all our merchants.
The company has a good reputation in this industry, and finally it tured out that choose them is a good choice.










