News
-

Heart disease needs a new drug – Vericiguat
Heart failure with reduced ejection fraction (HFrEF) is a major type of heart failure, and the China HF Study showed that 42% of heart failures in China are HFrEF, although several standard therapeutic classes of drugs are available for HFrEF and have reduced the risk of...Read more -

Targeted drug for the treatment of myelofibrosis: Ruxolitinib
Myelofibrosis (MF) is referred to as myelofibrosis. It is also a very rare disease. And the cause of its pathogenesis is not known. Typical clinical manifestations are juvenile red blood cell and juvenile granulocytic anemia with a high number of tear drop red blood cell...Read more -

You should know at least these 3 points about rivaroxaban
As a new oral anticoagulant, rivaroxaban has been widely used in the prevention and treatment of venous thromboembolic disease and stroke prevention in non-valvular atrial fibrillation. In order to use rivaroxaban more reasonably, you should know at least these 3 points....Read more -

Changzhou Pharmaceutical received approval to produce Lenalidomide Capsules
Changzhou Pharmaceutical Factory Ltd., a subsidiary of Shanghai Pharmaceutical Holdings, received the Drug Registration Certificate (Certificate No. 2021S01077, 2021S01078, 2021S01079) issued by the State Drug Administration for Lenalidomide Capsules (Specification 5mg, ...Read more -
What are the precautions for rivaroxaban tablets?
Rivaroxaban, as a new oral anticoagulant, has been widely used in the prevention and treatment of venous thromboembolic diseases. What do I need to pay attention to when taking rivaroxaban? Unlike warfarin, rivaroxaban does not require monitoring of blood clotting indica...Read more -
2021 FDA New Drug Approvals 1Q-3Q
Innovation drives progress. When it comes to innovation in the development of new drugs and therapeutic biological products, FDA's Center for Drug Evaluation and Research (CDER) supports the pharmaceutical industry at every step of the process. With its understanding of ...Read more -

Recent developments of Sugammadex Sodium in the wake period of anesthesia
Sugammadex Sodium is a novel antagonist of selective non-depolarizing muscle relaxants (myorelaxants), which was first reported in humans in 2005 and has since been used clinically in Europe, the United States and Japan. Compared with traditional anticholinesterase drugs...Read more -

Which tumors are thalidomide effective in treating!
Thalidomide is effective in treating these tumors! 1. In which solid tumors can thalidomide be used. 1.1. lung cancer. 1.2. Prostate cancer. 1.3. nodal rectal cancer. 1.4. hepatocellular carcinoma. 1.5. Gastric cancer. ...Read more -

Tofacitinib Citrate
Tofacitinib citrate is a prescription drug (trade name Xeljanz) originally developed by Pfizer for a class of oral Janus kinase (JAK) inhibitors. It can selectively inhibit JAK kinase, block JAK/STAT pathways, and thereby inhibit cell signal transduction and Related gene expression and activatio...Read more -
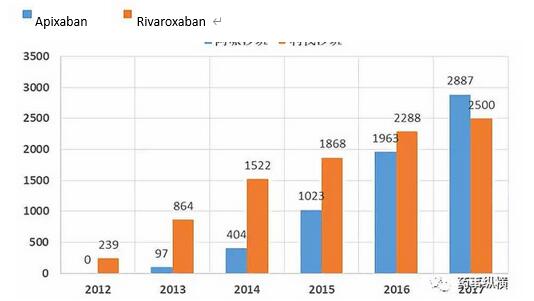
Apixaban and Rivaroxaban
In recent years, the sales of apixaban have grown rapidly, and the global market has already surpassed rivaroxaban. Because Eliquis (apixaban) has an advantage over warfarin in preventing stroke and bleeding, and Xarelto ( Rivaroxaban) only showed non-inferiority. In addition, Apixaban does not...Read more -

Guangzhou API exhibition in 2021
The 86th China International Pharmaceutical Raw Materials/Intermediates/Packaging/Equipment Fair (API China for short) Organizer: Reed Sinopharm Exhibition Co., Ltd. Exhibition time: May 26-28, 2021 Venue: China Import and Export Fair Complex (Guangzhou) Exhibition scale: 60,000 square meters Ex...Read more -
Obeticholic acid
On June 29, Intercept Pharmaceuticals announced that it has received a complete new drug application from the US FDA regarding its FXR agonist obeticholic acid (OCA) for fibrosis caused by non-alcoholic steatohepatitis (NASH) Response letter (CRL). The FDA stated in the CRL that based on the data...Read more










