Quality Inspection for Sofosbuvir Daclatasvir Dose - Sugammadex Sodium – CPF
Quality Inspection for Sofosbuvir Daclatasvir Dose - Sugammadex Sodium – CPF Detail:
| 舒更葡糖钠 | Sugammadex Sodium | 343306-79-6 | In-House |
Description
Sugammadex sodium is a synthetic γ-cyclodextrin derivative, and acts as a new reversal agent for neuromuscular block.
Generic Name: sugammadex (soo GAM ma dex)
Brand Name: Bridion
Sugammadex reverses the effects of certain medications that are given during surgical procedures to relax your muscles.
Sugammadex is used at the end of surgery, to help restore muscle function that has been blocked during surgery by the other medicines.
Sugammadex may also be used for purposes not listed in this medication guide.
It is used to reverse the effects of some drugs.
In Vivo
Injection of sugammadex has no significant effects on blood pressure or heart rate. After injection of the high rocuronium dose, the 90% recovery of the train-of-four ratio takes 28 min (SD 7 min) after saline, 26 min (SD 9.5 min) after 1 mg/kg sugammadex, and 8 min (SD 3.6 min) after 2.5 mg/kg sugammadex[1]. Sugammadex causes a rapid and complete reversal of rocuronium-induced neuromuscular block. The recovery time to train of four ratio=0.9 after spontaneous recovery is 14.4 min (SD=3.4 min; n=14). This is reduced significantly to 3.7 min (SD=3.3 min; n=4) with sugammadex 0.5 mg/kg and to 1.9 min (SD=1.0 min; n=4) with sugammadex 1.0 mg/kg[2]. The estimated half-life of sugammadex in Rhesus monkey is 30 (SEM=4.9) min[3].
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
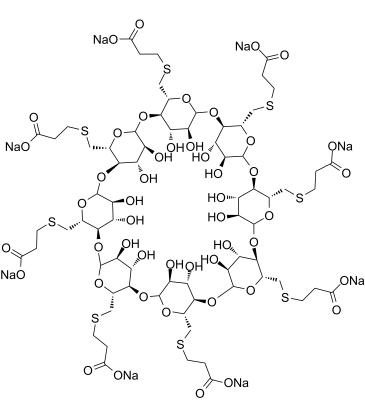
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
All we do is always associated with our tenet " Customer first, Trust first, devoting on the food packaging and environmental protection for Quality Inspection for Sofosbuvir Daclatasvir Dose - Sugammadex Sodium – CPF , The product will supply to all over the world, such as: Ireland, Kuala Lumpur, Islamabad, We focus on providing service for our clients as a key element in strengthening our long-term relationships. Our continual availability of high grade products in combination with our excellent pre-sale and after-sales service ensures strong competitiveness in an increasingly globalized market.
We always believe that the details decides the company's product quality, in this respect, the company conform our requirements and the goods are meet our expectations.











