Elagolix 834153-87-6
This medication is used by women to help relieve moderate to severe pain due to a condition called endometriosis.
May Treat: Endometriosis
Brand Names: Orilissa
Drug Class: LHRH (GnRH) Antagonists
Availability: Prescription Required
Pregnancy: Avoid use during pregnancy
Lactation: Consult a doctor before using
Elagolix is an orally bioavailable, second-generation, non-peptide based, small molecule compound and selective gonadotropin-releasing hormone (GnRH; LHRH) receptor antagonist, with potential hormone production inhibitory activity. Upon oral administration, elagolix competes with GnRH for receptor binding and inhibits GnRH receptor signaling in the anterior pituitary gland. This inhibits the secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone. In women, inhibition of FSH and LH prevents the production of estrogen by the ovaries. Inhibition of GnRH signaling may treat or prevent symptoms of sex hormone-dependent disease states.
Elagolix is an oral, nonsteroidal gonadotropin releasing hormone (GnRH) antagonist that decreases estrogen production and is used to treat painful forms of endometriosis in women. Elagolix therapy is associated with a low rate of serum enzyme elevations during therapy and has yet to be linked to instances of clinically apparent liver injury.
Elagolix has been used in trials studying the basic science and treatment of Endometriosis, Folliculogenesis, Uterine Fibroids, Heavy Uterine Bleeding, and Heavy Menstrual Bleeding. As of 24 July 2018, however, the U.S. Food and Drug Administration (FDA) approved AbbVie's elagolix under the brand name Orilissa as the first and only oral gonadotropin-releasing hormone (GnRH) antagonist specifically developed for women with moderate to severe endometriosis pain. It has been determined that endometriosis is one of the most common gynecologic disorders in the United States. In particular, estimates suggest that one in ten women of reproductive age is affected by endometriosis and experience debilitating pain symptoms. Moreover, women who are affected by this condition can suffer for up to six to ten years and visit multiple physicians before receiving a proper diagnosis. Subsequently, as Orilissa (elagolix) was approved by the FDA under priority review, this expedited new approval gives healthcare professionals another valuable option for treating the potentially unmet needs of women who are affected by endometriosis, depending on their specific type and severity of endometriosis pain.
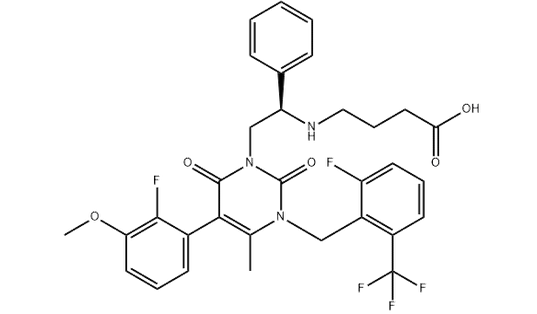
Chemical structure





Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room







