Rosuvastatin Calcium
| 瑞舒伐他汀钙 | Rosuvastatin Calcium | 147098-20-2 | In-House/CEP |
| RSM(Crystal) | 147118-40-9 | In-House | |
| RSM(Crude) | 147118-40-9 | In-House | |
| RSP | — | In-House | |
| RS-8 | 147118-36-3 | In-House | |
| RS-8-1 | 147118-37-4 | In-House | |
| TP-8 | 131466-61-0 | In-House | |
| TP-11 | 147118-35-2 | In-House | |
| TP-13 | 147118-39-6 | In-House | |
| RS-10 | 289042-12-2 | In-House | |
| RS-11 | 355806-00-7 | In-House |
Description
Rosuvastatin Calcium (BANM, JAN, USAN) is known as Rosuvastatin in the US. and a competitive HMG-CoA reductase inhibitor with an IC50 of 11 nM[1]. Rosuvastatin Calcium potently blocks human ether-a-go-go related gene (hERG) current with an IC50 of 195 nM, delayed cardiac repolarization, and thereby prolonged action potential durations (APDs) and corrected QT interval (QTc) intervals[2]. Rosuvastatin Calcium reduces the expression of the mature hERG and the interaction of heat shock protein 70 (Hsp70) with the hERG protein. Rosuvastatin Calcium is very effective in lowering low-density lipoprotein (LDL) cholesterol, triglycerides, and C-reactive protein levels[3].
Further information on drug naming conventions: International Nonproprietary Names.
Important Notice: The Drugs.com international database is in BETA release. This means it is still under development and may contain inaccuracies. It is not intended as a substitute for the expertise and judgement of your physician, pharmacist or other healthcare professional. It should not be construed to indicate that the use of any medication in any country is safe, appropriate or effective for you. Consult with your healthcare professional before taking any medication.
In the US, Rosuvastatin (rosuvastatin systemic) is a member of the drug class statins and is used to treat Atherosclerosis, High Cholesterol, High Cholesterol - Familial Heterozygous, High Cholesterol - Familial Homozygous, Hyperlipoproteinemia, Hyperlipoproteinemia Type IIa - Elevated LDL, Hyperlipoproteinemia Type IIb - Elevated LDL VLDL, Hyperlipoproteinemia Type III - Elevated beta-VLDL IDL, Hyperlipoproteinemia Type IV - Elevated VLDL, Hypertriglyceridemia and Prevention of Cardiovascular Disease.
Background
A selective, competitive inhibitor of HMG-CoA reductase, that is also antilipemic.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
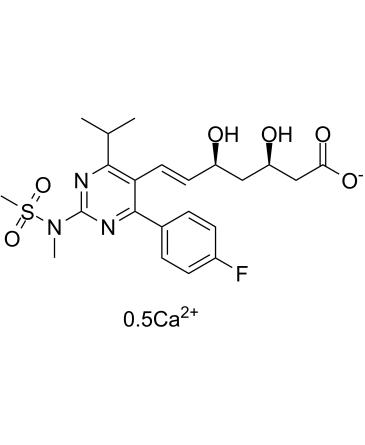
Chemical structure





Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room





